Needleless Glucose Monitoring for Seniors in 2026
In 2026, needleless glucose monitors are increasingly used in the United States for managing diabetes, especially among seniors. These devices measure glucose levels without finger pricks, potentially reducing discomfort and encouraging regular monitoring as part of daily diabetes care routines.
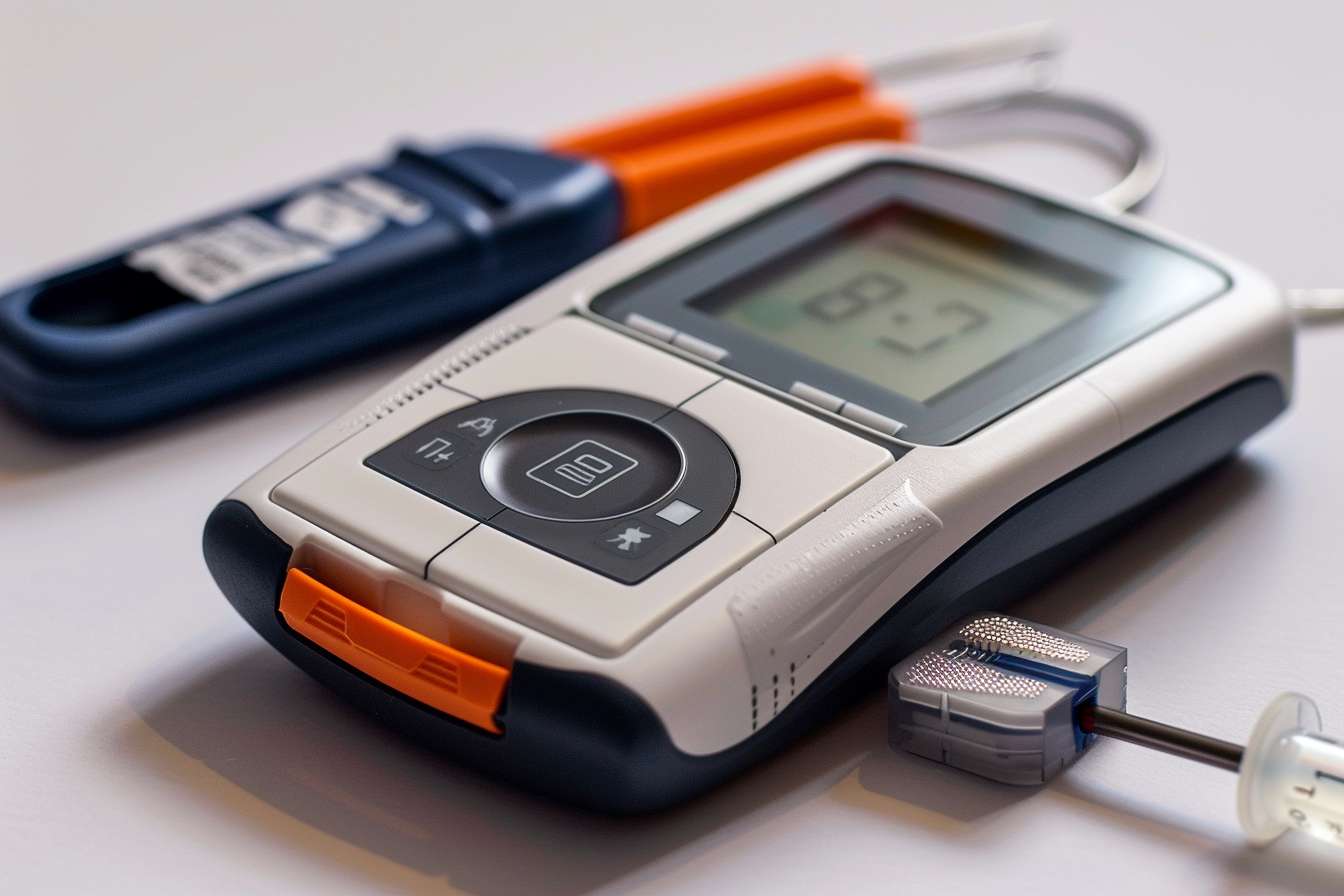
Overview of Needleless Glucose Monitoring Technology
Needleless glucose monitoring devices represent an alternative to traditional blood glucose testing methods, which typically require fingersticks to obtain blood samples. Instead, these devices use non-invasive technologies such as optical sensors or interstitial fluid measurement to estimate glucose levels. By avoiding direct blood sampling, needleless systems aim to reduce pain and simplify the glucose monitoring process.
There are several types of needleless glucose monitoring technologies currently under study or in use, including:
- Optical Sensors: These use light-based measurements to assess glucose concentrations under the skin.
- Transdermal Sensors: Devices that analyze interstitial fluid through the skin without puncturing blood vessels.
- Sweat-based Sensors: Experimental devices that attempt to detect glucose in sweat.
Most currently available continuous glucose monitors (CGMs) approved for use still require a small subcutaneous sensor insertion but are considered minimally invasive compared to multiple daily fingersticks. Moving fully to needleless, non-invasive methods remains a focus within medical research and device development.
Importance for Seniors in Diabetes Management
Managing diabetes in seniors involves unique challenges, including reduced manual dexterity, vision limitations, and multiple coexisting health conditions. Traditional glucose monitoring techniques can be difficult to perform regularly and accurately for many older adults.
Needleless or minimally invasive monitors may offer the following advantages for seniors:
- Reduced Pain and Discomfort: Minimizing or eliminating finger pricks can help older adults with sensitive skin or other health issues.
- Ease of Use: Simplified device handling and automated measurements reduce the burden of self-monitoring.
- Increased Monitoring Frequency: Less invasive measurement may encourage more frequent glucose checks, helping better diabetes control.
Despite these benefits, some seniors may face challenges such as adapting to new technology interfaces, costs, or device maintenance.
Technological Developments in 2026
As of 2026, continuous glucose monitors remain widely used in the United States, with ongoing advancements focusing on accuracy, sensor lifespan, and data connectivity. The most common CGMs minimally infringe the skin by inserting a small sensor, yet data transmission and reading can be done via smartphones or dedicated receivers.
Research into fully non-invasive glucose monitoring continues but has not yet produced commercially widespread options meeting clinical accuracy standards. Some devices on the market utilize optical or electromagnetic techniques but may have limitations in reliability or regulatory approval for sole diabetes management.
Regulatory bodies such as the U.S. Food and Drug Administration (FDA) continue to evaluate new glucose monitoring technologies, emphasizing safety, accuracy, and usability for all populations including seniors.
Medicare and Insurance Considerations in the United States
In 2026, Medicare Advantage plans and some private insurers in the United States generally cover certain continuous glucose monitoring devices for eligible beneficiaries with diabetes. Coverage may include sensors, transmitters, and readers needed to operate these systems.
Key points relevant for seniors include:
- Medicare Coverage: Medicare Part B and some Medicare Advantage plans may cover CGMs if prescribed, subject to medical necessity criteria.
- $0 Copay Options: Certain plan members obtaining CGM supplies through in-network pharmacies can receive these with no copay, depending on plan rules.
- Prior Authorizations: Some CGM devices require prior authorization prior to coverage approval.
Patients and caregivers should consult plan resources or healthcare providers for guidance on coverage and device options.
Accuracy and Clinical Use
Glucose monitoring accuracy remains critical in managing diabetes safely. Needleless and minimally invasive systems provide frequent or continuous data to support treatment decisions but should be used according to clinical guidelines.
Limitations of needleless monitors as of 2026 may include:
- Potential measurement delays compared to blood glucose due to interstitial fluid lag time.
- Calibration requirements for some devices.
- Influence of environmental factors or skin conditions on sensor readings.
Healthcare providers recommend confirming significant glucose values with traditional methods if symptoms do not match device readings.
Challenges and Considerations for Seniors
While needleless and continuous glucose monitoring technologies address many barriers to glucose monitoring in seniors, challenges remain:
- Technical Literacy: Some seniors may require assistance or training to operate devices and interpret data.
- Device Maintenance: Regular replacement of sensors or batteries, and managing associated equipment, may be cumbersome.
- Skin Sensitivity: Adhesive patches used to attach sensors can cause irritation in some users.
- Data Privacy: Connectivity features, including smartphone apps, involve handling health data with appropriate privacy safeguards.
Efforts to provide user-friendly interfaces and support resources aim to increase adoption and effective use among older adults.
Typical Costs in United States (2026)
When considering needleless or continuous glucose monitors in the United States, typical price ranges include:
- Basic Option: Approximately $35 to $75 for single-use or short-term sensors; suitable for occasional monitoring or trial use.
- Standard Option: Between $300 to $600 per month for sensor supplies and required transmitters; includes devices like earlier generation CGMs.
- Premium Option: Up to $1000 or more per month for advanced CGM systems with longer sensor life, integration with insulin pumps, and enhanced data analytics.
Insurance coverage significantly influences out-of-pocket costs. Medicare and some commercial plans may cover part or all device expenses for eligible individuals.
Future Outlook
Continued research aims to improve non-invasive glucose monitoring technologies. Goals include:
- Enhancing sensor accuracy to meet or exceed current CGM performance.
- Increasing sensor wear time to reduce replacement frequency.
- Developing devices that do not require skin puncture at all.
- Improving integration with digital health platforms for comprehensive diabetes management.
Monitoring innovations hold potential to support better health outcomes in the aging population with diabetes. However, clinical validation and regulatory approval processes will determine the technologies broadly available to seniors in the coming years.
Summary
Needleless glucose monitoring systems provide an alternative approach to traditional blood glucose testing, particularly relevant for seniors managing diabetes in the United States. While fully non-invasive devices are under development, current continuous glucose monitors offer minimally invasive options with benefits related to comfort and ease of use. Understanding device capabilities, costs, insurance coverage, and clinical considerations can support informed decisions about diabetes management strategies in 2026.




